Bal-tec™ Home Rusty Balls
Rusty Balls

Rust, or corrosion, of precision balls can be a serious problem when the balls are made of some common bearing alloy steels. The ambient environment in the storage area can be a major contributor to the presence of corrosion (rust) on precision balls. About 50 years ago we began storing our small overruns of chrome steel (52100) balls in small, screw-top glass-jars in Roto-Bin shelving. We would put the clean, dry balls in the jars and put a squirt of oil over them.
We began experiencing a rust problem that turned out to be caused by the moisture in that small volume of air above the balls inside the jars. Over many months or even years of storage, the temperature changes from day to day caused a precipitation of minute water droplets that gradually built up over time and caused serious corrosion (rust) on the surface of these balls. We finally had to scrap this entire inventory of balls.
We now use the same basic system, but we top off the glass jars with oil to eliminate this pocket of moisture-containing air. We have since had no further corrosion problems. Let’s look at the multiple types of ball corrosion and their probable causes. There are two general forms that rust, or corrosion, takes on precision balls:
Cause Number One: shows up as a surface discoloration.
This is usually large gray to black areas that appear to be stains. In situations where the rusting has just occurred, the stains can be yellow, brown, or even reddish brown. All degrees of this condition are environmentally induced. Somewhere along the line, the “clean dry” balls have been exposed to moisture. Another version of this same condition that can manifest itself in the same kind of staining, is that the balls were coated with a classical rust-inhibitor that consists of a polar form of petroleum product, dissolved in a solvent. When this solvent subsequently evaporates, it leaves a layer of rust-inhibitor on the product.
The problem is that over time this petroleum product undergo chemical changes (polymerization) that includes some water vapor extracted from the surrounding air and this moisture actually causes rust-staining of the balls. One of the incidents that has become folklore in our company was an incident that occurred late one unusually-humid August night, several years ago.
It was almost eleven o’clock in the evening, and we were struggling to clean some large diameter, ultra-precise 52100 chrome steel balls for shipment the next day. We were using a three stage cleaning process in a large ultrasonic cleaner. We were using isopropyl alcohol and a post process blow-drier.
Everyone in the crew was dead tired when someone dropped one of these large-diameter balls out of its Teflon cradle, into the glass-lined tank of the ultrasonic cleaner and smashed the glass liner to smithereens. We basically gave up for the evening and went home. When we came in early the next morning we were dumbfounded! The top half of every one of these absolutely clean, absolutely dry balls was a deep brown color, exactly to the hemisphere. During that eight-hour period, moisture had settled out of the humid evening air and rusted every single one of these balls a deep brown color. The balls were dimensionally near their high limit, so we returned them to the ultra-precise department, where it took twenty millionths of an inch to remove every single trace of rust. This was only, an amazing, ten micro-inches per side. We went ahead and removed another twenty microinches to be safe, and we were able to ship the finished balls to the customer, the next day.
My father use to say that chrome steel would rust while you watched and I now know that he was right.
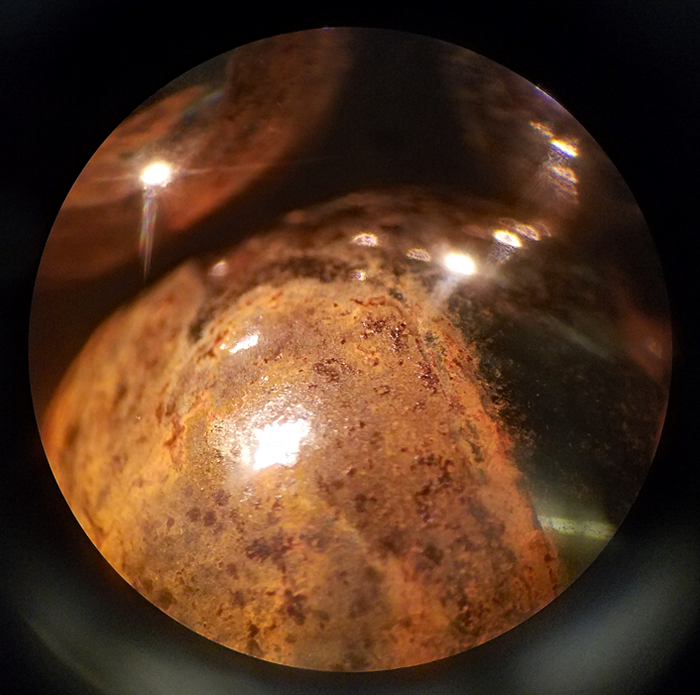
The Number Two Cause: and a far more sinister form of rust (corrosion) problem, is rust-pitting.
This condition manifests itself as very occasional dots of rust. These dots are frequently a brown gray color in the early stage and gradually turn black. This condition seldom occurs in balls under three quarters of an inch (19mm) diameter.
It is our experience that this condition always has a chemical origin going back to the alloy production stage.
When these small dots of rust are spectrographically analyzed with a scanning electron, or ion beam machine, they are invariably found to contain rust accelerating chemicals such as chlorine or sulfur. When parts made of a low alloy steel with thick cross section, such as the ever popular 52100 alloy (chrome steel), are heat treated, they must be severely quenched, usually in a water based salt solution (brine) in order for them to be hardened. Based on a lot of experience, we feel that the most probable cause of this form of corrosion is that minute amounts of a corrosion accelerator has lodged into small pores or micro cracks in the ball’s surface. If you think that steel, even rolled and forged steels. are one-hundred percent dense, you only have to look at the surface of a metallurgically polished piece of this metal under a 600 power metallurgical microscope. There are pores and voids everywhere.
Outside of the pure probabilities, the fact is that this form of corrosion only occurs on large balls that have been brine-quenched and that if one of these spots is vigorously rubbed on a piece of cloth the spot is reduced to a microscopic black dot that literally requires a microscope to see. The area that made up the original rust spot is now an almost invisible dot of lightly etched clean metal. This dot represents the minute volume of ferrite that went into forming the Fe2O2 and Fe2O3 as well as ferric chloride and similar compounds that formed the original rust dot.
If these brief observations impress you with how important control is in preventing rust of precision balls we have achieved our goal.
